Background: The 5-year overall survival for pediatric blood cancer (i.e., leukemia, lymphoma) is greater than 85%. However, blood cancers and their treatment predispose survivors to the risk of late effects and premature mortality. This study examines the association between the receipt of a personalized survivorship care plan (SCP) and mortality among survivors of childhood blood cancer.
Methods: This retrospective cohort included survivors who were treated for a newly diagnosed blood cancer at Children's Healthcare of Atlanta (CHOA) between 2002-2016 and in remission for ≥2 years post-treatment (i.e., eligible to be seen in Aflac Cancer Survivor Clinic). Descriptive analyses were conducted to examine SCP receipt and mortality through 12/31/2020 based on linkage to the National Death Index. The cumulative incidence of death was estimated from the time of eligibility for survivor clinic until the date of death, 12/31/2020, or five years (y) post-eligibility date, whichever occurred earlier. A multivariable Cox proportional-hazard model was used, with SCP receipt as a time-dependent variable, to estimate the association between SCP receipt and 5-year mortality, controlling for age at diagnosis, sex, race/ethnicity, and cancer therapy exposure.
Results: Among the 1,239 eligible survivors identified, 982 (79.3%) received a SCP at a median time of 0.50 y (IQR: 0.21, 0.84 y) from eligibility for survivor clinic. Recipients of a SCP (vs. non-recipients) were more likely to be female (44.8% vs. 38.1%; p=0.055), non-Hispanic White (56.6% vs. 47.5%; p=0.006), and younger at the initial cancer diagnosis (mean ± SD age: 8.4 ± 5.4 vs. 11.0 ± 5.8 y; p<0.001). Receiving a SCP differed by cancer type: 82.3% of patients diagnosed with acute lymphoid leukemia (ALL), 86.3% of acute myeloid leukemia (AML) patients, and 80.6% of other leukemia patients received a SCP vs. 68.6% of Hodgkin lymphoma patients and 76.1% of non-Hodgkin lymphoma patients (p<0.001). Therapy exposure differed among SCP recipients, with 75.6% receiving chemotherapy alone, 12.9% chemotherapy and radiation therapy, and 11.5% stem cell transplantation (SCT)
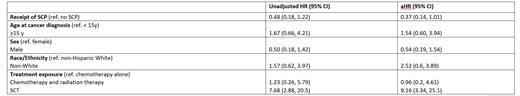
The unadjusted 5-year survival was not significantly different between survivors who received a SCP and those who did not (98.8% [95% CI: 98.1, 99.6] vs. 96.4% [93.9%, 98.9%], p=0.065). In multivariable analysis, receipt of a SCP was marginally associated with a lower risk of death (adjusted hazard ratio [aHR]: 0.37 [95% CI: 0.14, 1.01], p=0.053; Table 1). Additionally, receipt of SCT (aHR: 9.16 [95% CI: 3.34, 25.1], p<0.001) was associated with a higher risk of death.
Conclusions: Early evaluation of our cohort eligible for a survivorship clinic suggests an association between the receipt of a SCP and a higher adjusted 5-year survival in pediatric blood cancer survivors. Receiving an SCT remains an independent risk factor for mortality. Longitudinal follow-up of this cohort allows for the assessment of mortality trajectory and pattern. Capture of healthcare utilization will inform the impact of survivorship care and further define lifetime risk after contemporary treatment.
Disclosures
Castellino:SeaGen Inc.: Other: Scientific Advisory Committee - No honoraria, Research Funding; Bristol Meyers Squibb: Honoraria, Other: Scientific Advisory Committee. Effinger:Pfizer: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal